
Heart Failure (HF)
One of the leading causes of death in the developed world
- Affects 5.7 million Americans
- Affects 10 million Europeans
- As condition worsens, quality of life diminishes

A Growing Problem
Number of people diagnosed with HF is increasing and projected to rise by 46 percent by 2030, resulting in more than 8 million people with HF due to
- Increasing sedentary population
- Epidemics of diabetes and obesity from diet both contribute
- Growing population of the elderly are particularly susceptible
- $8-15 billion per year is spent on hospitalization in the US due to HF

Treatment Methods
When lifestyle changes, medication and surgical intervention are unable to slow progression, heart transplantation is the only permanent treatment modality.
- Limited donor hearts available (~2,500 – 3,000 per annum in US)
- Waiting list eligibility excludes elderly and those with other health conditions
- For those on the waiting list, wait times can be lengthy.
OUR MISSION
To increase longevity, alleviate the suffering and
improve the quality of life of patients suffering
from heart failure.
Heart Failure
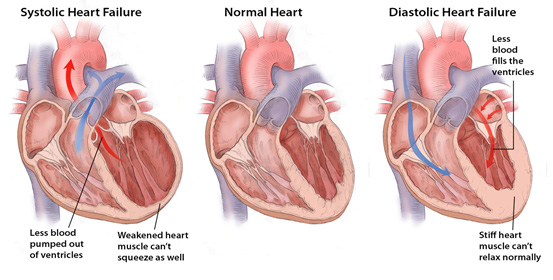
Heart failure (HF) is a progressive condition in which the heart’s muscle becomes weakened after injury. There are two types of left-sided HF as well as right sided failure:
- systolic failure occurs when the left ventricle loses its ability to contract normally; the heart can’t pump with enough force to push enough blood into circulation;
- diastolic failure which occurs when the left ventricle loses its ability to relax normally (because the muscle has become stiff), and the heart can’t properly fill with blood during the resting period between each beat;
- right-sided HF occurs when the right side loses pumping power, causing blood to back up in the body’s veins. This usually causes swelling or congestion in the legs, ankles and swelling within the abdomen.
Heart Failure Treatment Modalities
Current treatment for HF includes (generally in order of the progression of the symptoms)
- Lifestyle Changes
- Diet & Exercise
- Medication
- First line of defense against heart failure
- Provides limited benefit in advanced heart failure patients
- Usually does not treat underlying disorder
- Only slows progression of the disease
- Current best-practice drug therapy for HF has low efficacy for end-stage HF patients
- Surgical repair/reshaping
- Mechanical Circulatory Support
- Ventricular Assist Devices (“VADs”). Current VADs are used for patients with severe heart failure including those with irreversible damage who cannot be treated effectively by medical or surgical means other than transplant.
- Total Artificial Heart (TAH)
- Cardiac Transplantation
What is a Ventricular Assist Device (VAD)?
A VAD is a mechanical circulatory system employed to partially or completely substitute for the function of a failing heart, to increase blood flow. VAD’s have been designed and configured for either temporary use while hospitalized or implanted for temporary or long-term outpatient treatment.
There are several clinical applications for VADs:
- “bridge-to-transplant” – implantation is temporarily to support a heart failure patient awaiting a heart transplant;
- long-term support towards a “destination therapy” for patients who are not eligible for a heart transplant;
- “bridge-to-recovery” in instances where a patient’s heart failure is short-term and where a VAD can be implanted for a few weeks or months to assist the heart during its recovery period.

Current generation VAD’s include:
- An implanted pump that is positioned outside the heart that pulls blood from the left ventricle through a tube that has been implanted in the heart;
- A tube on the opposite side of the pump that re-routes the blood into the aorta above the aortic valve;
- The pump is connected to a driveline that exits the body through the abdomen;
- The driveline is connected to an external powerpack and controller unit that the patient must wear 24-7.
Shortcomings of Current VADs
While the current generation of VADs have extended lives and enhanced the quality of life for patients there remains a need for a new generation of VADs to drive significant improvement in clinical outcomes resulting in increased longevity and enhanced quality of life for HF sufferers.

Major drawbacks
- Highly invasive implant procedure
- Removal of heart tissue permanently alters heart architecture
- Continuous blood flow is not physiologic
- Patient is tethered to bulky, inconvenient external power and controller unit 24-7
- Application limited to those with advanced, severe HF

Adverse Effects
- Incidence of complication requiring hospitalization:
- gastrointestinal and/or cerebral bleeding from non-physiologic blood flow
- driveline infection
- depletion of clotting factors
- thrombus formation over non-physiologic surfaces
- Device failure resulting in mortality

Limitations in Use
- Restricted to left ventricular dilated cardiomyopathy
- Improvement in left sided assist may precipitate or worsen right side failure
- Problematic to use in the setting of an acute myocardial infarction
MI-VAD: The Next Generation VAD
The MI-VAD pump comprises 3 primary assemblies that combine to offer a minimally invasive, non-cannula, fully internal, destination treatment and bridge to transplant for Class III and IV heart failure sufferers. Located in a supra-valvular position in either the aorta or pulmonary artery the device is equally effective to augment, and restore cardiac function in the left or right ventricles and for cases of either dilated or restricted cardiomyopathy. MI-VAD represents a paradigm shift in VAD technology and a conceptual approach which is truly novel.
MI-VAD Competitive Advantages
We have completed device proof of concept through prototype pump design, build and test. We also achieved surgical proof of concept from successful acute animal studies. In these studies, our prototype pumps were implanted above the aortic and pulmonary valves in a live animal model, and the key concepts which distinguishes our device were demonstrated:
- Effective for both dilative and restrictive cardiomyopathy
- Targeted flow rates and pressures achieved
- Pump speed variation successfully synchronized to animal’s cardiac rhythm
- Reduced power requirements
- Unloading of dilated heart and good cardiac output demonstrated

We are continuing with development activities including refinement of our pump design and its integration with:
- Internal power supply and controller
- ECG gating operating system
- Transcutaneous charging system and diagnostic interface
- Surgical toolkit
We will conduct acute and chronic animal studies leading to a design freeze necessary to support investigational human trials.









